Recently, the Hydroxyapatite-Coated Porous Titanium Alloy Interbody Fusion Device (WedoCageTM) developed by Wedo Bio-Medical Technology Co, Ltd. (hereinafter referred to as " Wedo"), has officially received market approval after obtaining the Class III Medical Device Registration Certificate from the National Medical Products Administration (NMPA).
Sidebar
Tooling

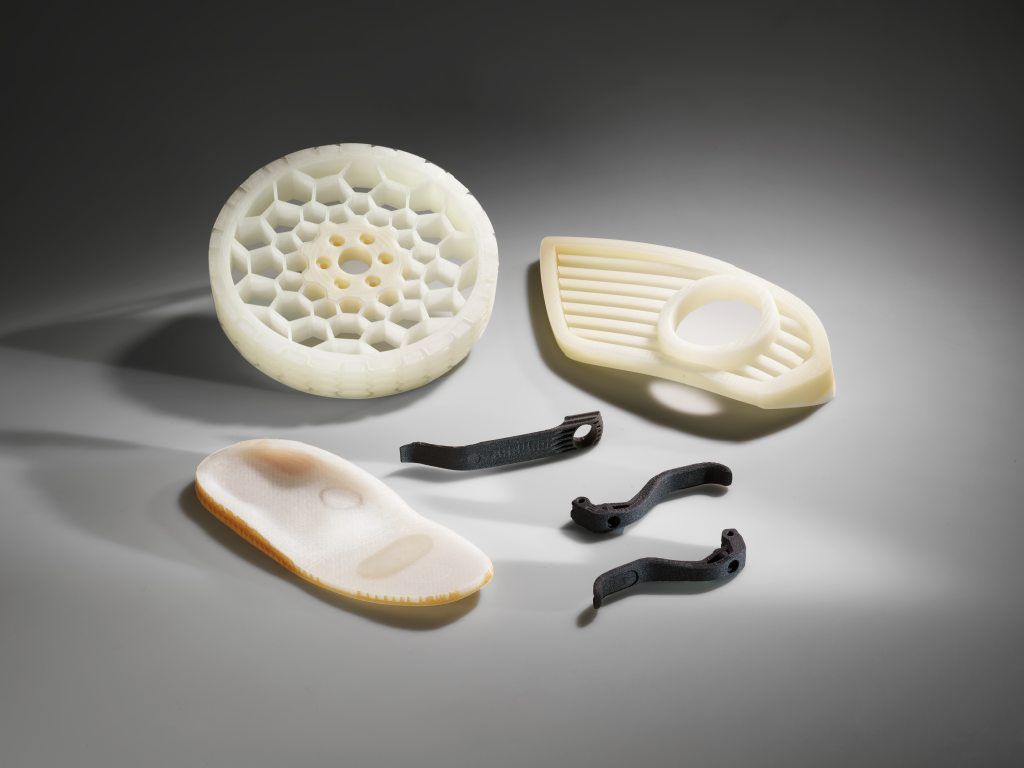
Additive processing of high-temperature plastic, carbon fibres and liquid silicone
• Freeformer 750-3X: High-temperature version for components made of certified Ultem original granules
• TiQ: Compact 3D printers produce robotic grippers from reinforced filament
• LiQ: Reliable processing of liquid silicone

World first: voxeljet unveils groundbreaking cold IOB 3D printing technology
voxeljet AG (NASDAQ: VJET), a global leader in industrial 3D printing solutions, has unveiled a groundbreaking innovation at the prestigious GIFA 2023: the new cold IOB (Inorganic Binding) 3D printing technology. With this unique process, moulds and cores for the foundry industry can be produced for the first time from sand and an inorganic binder without the need for microwave treatment.

3D print implants in PEEK with 3D Systems
The EXT 220 MED is the new name for the Kumovis R1 as part of our growing extrusion printing class of printers. This platform is a game-changer in medical device manufacturing, enabling 3D printing of implants and instruments using high performance polymers such as PEEK and Radel® PPSU. Now, thanks to the ingenuity of our engineering teams, we are excited to announce a performance upgrade to allow up to 30% faster throughput.

Easy, flexible automation beyond the machine itself with the tog.519
The tog.519 cobot from Schubert is ready for series production. And, for the very first time at this year’s interpack, the packaging machine manufacturer will be showing the unimagined possibilities that consumer goods manufacturers can now exploit with this fast and simple automation solution. Three cobots will be presented in action, two of them on a filling machine for cosmetics manufacturer Börlind.

Evonik’s VESTAKEEP i4 3DF PEEK filament biomaterial was used in the first US surgeries for 3D printed spinal implants
VESTAKEEP® i4 3DF is the world’s first implant-grade filament based on PEEK for use in medical 3D printing of human surgical implants
Curiteva’s world’s first 3D printed spinal implants for commercial use are based on Evonik’s VESTAKEEP® i4 3DF biomaterial filament
Combination of Curiteva’s technology and Evonik’s cutting edge material offers the possibility of enhance integration and healing for spinal surgery

Express manufacturing for drawing parts
Contract manufacturer sets new standards for turned parts

Optomec Adaptive DED Repair Capabilities Presented at ASTM Additive Manufacturing Workshop
Focus on DED Adaptive Repair for Turbine Engine Components

AddUp Celebrates the Grand Opening of Their New Additive Manufacturing Facility: The Tooling Competence Centre, in Aachen, Germany
AddUp, global metal additive manufacturing OEM, is excited to officially open the doors to their new Tooling Competence Centre in Aachen, Germany. A joint venture created by Michelin and Fives in 2016, AddUp is headquartered in Cébazat, France. The Tooling Competence Centre is located within the WBA Tooling Academy in Aachen, Germany.

Stratasys Wins Multi-System Deal from German Manufacturer for Mass Production H350 3D Printers
Götz Maschinenbau further validates success of Stratasys manufacturing strategy as it grows its fleet to 6 SAF technology printers to meet growing demand for end-use parts

Stratasys Signs Agreement with Ricoh USA, Inc. for Print-On-Demand Medical Models
Collaboration increases access to 3D-printed anatomic models for hospitals and clinics.

 Deutsch (Germany)
Deutsch (Germany)  Polski (PL)
Polski (PL) 







