Fully executed memorandum of understanding (MOU) leverages both organizations’ highly sustainable production technologies to manufacture spherical powder
Sidebar
Consumables

Addition of New Stratasys Materials Reinforces Commitment to End-use Production and Manufacturing-grade Prototyping
Company announces four new materials for the P3™ DLP platform and two new materials, including color enhancements, for the F900
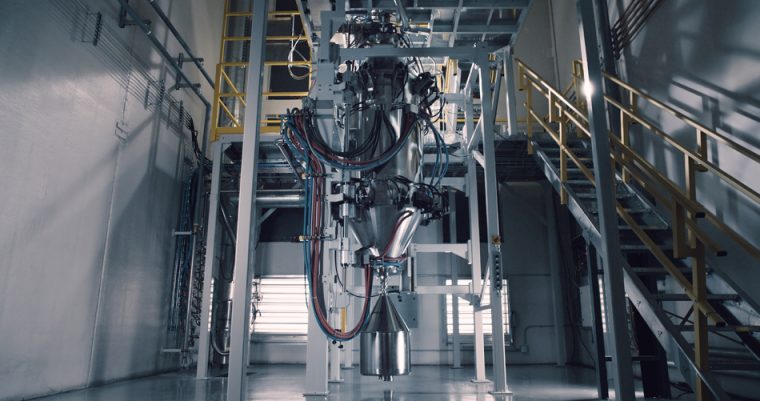
Equispheres and Aconity3D achieve remarkable productivity improvements using laser beam shaping and NExP-1 high-performance AM powder
Equispheres Inc. and Aconity3D are once again breaking new ground in metal additive manufacturing, achieving build rates about 9x greater than industry norms for aluminum powder and laser powder bed fusion.

Printer/Powder Combination Makes Metal Additive Manufacturing More Accessible to Smaller Enterprises
Printer/Powder Combination Makes Metal Additive Manufacturing More Accessible to Smaller Enterprises
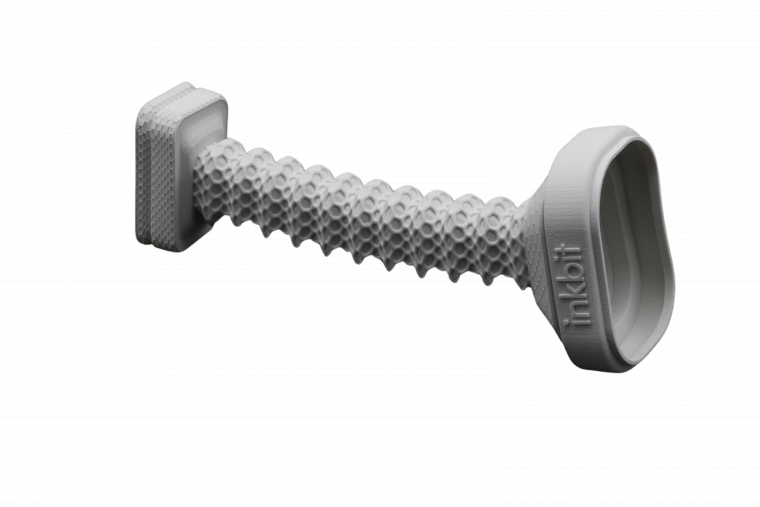
Inkbit Introduces TEPU 50A, a new soft-medium durometer elastomer
Empowering manufacturers to 3D print elastic components that require sealing, accuracy and design freedom

Industrial 3D printing - Advances with fiber-reinforced polyamide for powder bed processes
Industrial 3D printing requires plastic materials that, as printed components, correspond to the performance level of injection-molded components. This is the key to finding application for special components or in the spare parts market without major qualification effort and design adjustments. The standard materials in the plastics industry primarily include PP GF10, PP GF30 or PA6 GF30 - i.e. fiber-reinforced materials.
At Formnext 2023, Elkem will display its extensive portfolio of silicone solutions for this innovative technology
At Formnext 2023, the leading event for Additive Manufacturing and industrial 3D printing in Frankfurt, Elkem will display its extensive portfolio of silicone solutions for this innovative technology.

Henkel Loctite 3D Printing launches novel innovative materials for industrial applications at Formnext
Henkel will introduce four new products to its growing portfolio of photopolymer resins for 3D Printing at Formnext 2023 in Frankfurt. The novel innovative materials are set to make a significant impact in many types of industries, offering exceptional performance and versatility.

Unleashing Innovation and Endless Possibilities: Discover Elkem at the CIIE in China
With a rich history spanning over 70 years as an innovative force in silicone technology, Elkem is set to unveil a series of groundbreaking products at the 6th China International Import Expo (CIIE) from November 5 to 10, 2023.

Henkel introduces new medical grade wearables device light cure adhesives
As a leading manufacturer of advanced adhesive solutions for the medical industry, Henkel has launched a novel medical grade light cure adhesive designed for devices that are to be worn on the body. The new product is formulated without IBOA (Isobornyl Acrylate) or any other known skin sensitizing monomers. The adhesive is also formulated to conform to EU MDR 2017 regulations concerning use of CMR (carcinogenic, mutagenic, or toxic for reproduction) substances in medical devices making it a robust option for new designs.

 Deutsch (Germany)
Deutsch (Germany)  Polski (PL)
Polski (PL) 









